Process Pending: Glaciers as Archives pt. 1
Donate to a fundraiser to support Mahmoud Khalil here.
Personal archives are in. ⟵These archives engage thoughtfully and beautifully with history, identity, culture, etc. I, on the other hand, have a collection of almost every Campina (2%) yogurt container we have emptied since moving to the Netherlands. Is it an archive if all the items are the same, unchanged, and available in stores now? I suppose they are all stamped with different expiration dates. Anyways, drop me a line if you have any crafty ideas for what to do with 50 small buckets.
You know what else are archives? smiles and shrugs weakly at this forced transition
glaciers !
Glaciers are both memory keepers and autobiographers. The air and water that make up glaciers are archives of past climate, and their crystal structure a history of past stress and strain. As they form, flow, and disappear, they carve messages into the landscape, and we can read these geologic messages to understand their habits and the extent and mode of their past existence.
The first post on glaciers as archives should have been part 0; now it is. To introduce it, I wrote:
… calling a glacier an archive is so literal, it is really a description instead of a metaphor. But it offers a different point of view from which to ask questions – e.g., how does a glacial system 'select’ which data to preserve. I am interested in whether these questions end up giving us the same physical explanations, or whether they can add additional perspective. Is this a way of recognizing agency in physical systems?
Part 1 (here, now) introduces all the ways glaciers are archives. If you’re just interested in recommendations and/or an educational exercise on speculative ice cores, scroll on down to the bottom of the post.
The basics
Glaciers form when snow falls and sticks around. Over time, compressed under the weight of each subsequent snowfall, snow densifies into firn and then after some time (20-200 years or so) into glacial ice. So glacial ice is, more or less, made up of cake-like layers of old, squashed snow, which increase in age with depth.
Glaciers are, or maintain, archives in five main ways:
Water isotopes (ice is mostly lightweight oxygen and hydrogen)
Trapped air bubbles (sample-packs of old atmosphere)
Preservation of events (e.g., volcanos) and organic material (e.g., pollen, animal remains)
Ice fabric (crystalline memory)
Glacial geology (a glacier’s autobiography)
During the transition from snow to ice, archives 2-3 are created. The first kind, water isotopes, begins even before the first snowflake touches the ground.
1. Water isotopes
“Snowflakes are letters sent from heaven.” - Ukichiro Nakaya
The archiving begins the moment a snowflake forms.
Water is made up of hydrogen and oxygen. All elements are defined by a certain number of protons, but can have different numbers of neutrons—these are called isotopes. So hydrogen can have one proton, no neutrons (H1), one proton one neutron (H2), etc. The number to the right indicates the sum of the number of protons and neutrons. Oxygen has three stable isotopes, O16, O17, and O18. So always 8 protons, but between 6 and 8 neutrons5. These occur naturally. Most oxygen on our planet is O16, and we know the ratio of relative occurrence of O16:017:O18 pretty well for at least some of geologic history.
The more neutrons an isotope has, the heavier it is. More stuff=more mass. In the hydrologic cycle, water molecules with light isotopes (O16) evaporate from the ocean slightly faster. Once in the air, heavier isotopes (O18) fall out sooner. At colder temperatures, this effect becomes more pronounced. So when it is cold, in global climate terms, fewer of the heavy isotopes have evaporated into the air to become rain and snow, and more of the heavy isotopes fall out as rain before they reach the glaciers and ice sheets.
This means snowflakes, and glacial ice, are rich in O16 and poor in O18. The colder it is, the greater this ratio, and vice versa as it warms. We can analyze this ratio using mass spectroscopy from ice core samples. Ice cores are collected using drills that cut into the ice. Ice samples are brought up a meter or two at a time, and sliced like a Michelin-star cucumber for analysis. Here’s what a piece of an ice core looks like:
And here are all the ways it is sliced and diced for analyses:
2. Trapped air bubbles
Firn is the intermediary step between snow and glacial ice. Again, it’s just a fancy word for old snow. Importantly, like a snow cone—it’s still porous—so sugary liquid and the air in the atmosphere can reach to the bottom of the cup (a.k.a. the bottom of the firn pack). Here’s a close up of what firn looks like using scanning electron microscopy— lots of tubes and tunnels.
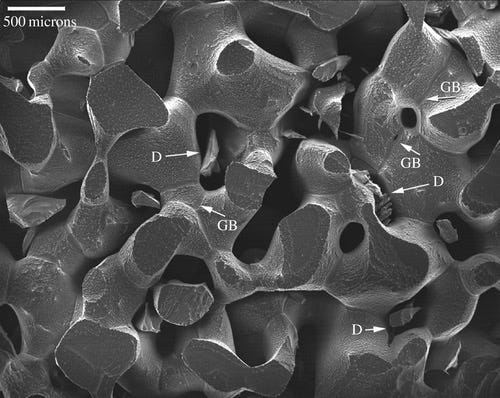
This means that the air in the firn is for the most part the same as the air in the atmosphere. Fresh snow is 100-300 kg m-3. Once the firn gets to a density of ~830 kg m-3, the tunnels close, the tubes seal, and the air from the current atmosphere becomes trapped in bubbles in the ice. These are little grab bags of the past atmosphere, and bubbles deeper down in the ice contain older air. From this air we can look at things like CO2 concentration.
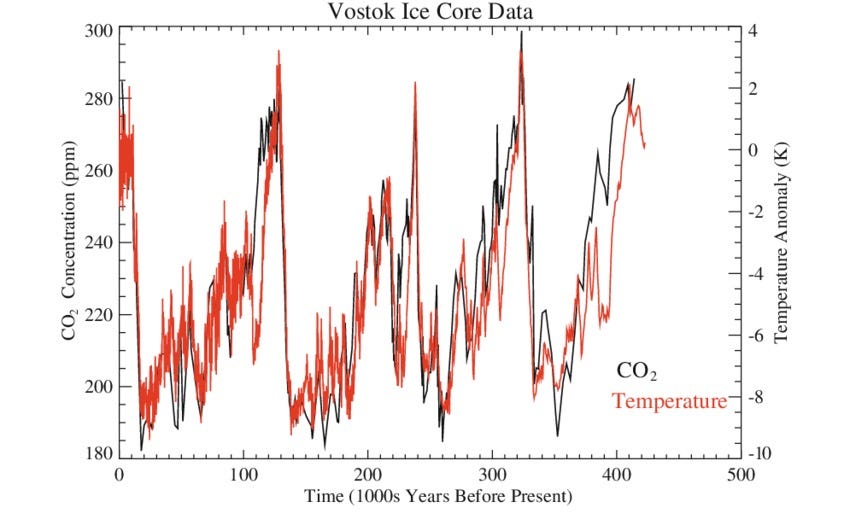
So, now we have a proxy of what the temperature was from the O16:O18 ratio from the snowflakes, and little samples of atmosphere from (more or less) the same time.
These two measurements from the Vostok Ice Core are (more or less) the first records that confirmed the relationship between CO2 and global temperature over the last 400,000 years.
3. Preservation in the ice
A glacier is built up layer by layer. When something lands on the surface of the glacier, it can be buried by subsequent snowfall and is preserved within it. In ice cores, we can see things like large volcanic eruptions—you can see the ash layer in the first image of the ice core sample. These events help constrain the age of the ice, because we can compare them to other records. Also in alpine glaciers, rather famously, we get well-preserved specimens and/or genetic material of humans, plants, and animals.
4. Ice fabric
Glaciers are made up of frozen water, which forms into a hexagonal crystal structure when it freezes. That’s why snowflakes have hexagonal symmetry.
A glacier’s hexagonal crystal structure, blown up to a macro scale, might look something like the basalt columns in Iceland.
This asymmetry of a hexagonal crystal structure (symmetric when you rotate it along the column axis, not symmetric when you flip it sideways) gives ice some interesting properties. Namely, when you stress the ice, those crystals rotate and reorganise so that the columnar axis aligns with the direction of compression (along the same axis the glacier is being squeezed). If you only push down on the ice, all the ice crystals point up/down. When you start squeezing ice through mountain ranges—things get interesting. We can see ice crystal rotation in ice cores and in some kinds of radar data. We care about the orientation of the crystals because 1) it tells us about the past stresses the ice underwent and 2) it changes the way ice will respond to present and future stresses. Ice remembers past stress and changes how it behaves depending on it! Same as us humans, no?
5. Glacial geomorphology, or, what a glacier writes on the landscape
Finally, glaciers write their autobiographies into the landscaps. Alpine glaciers scoop out u-shaped valleys, carve arétes, and whittle cirques. They leave behind moraines (hillocks of debris) to tell us how far they extended, what shape their fronts were, how wide they were. Drumlins and megascale lineations can tell us about how water flowed underneath the glaciers and how fast the glaciers flowed. Even larger features, like the Hudson River in New York, were formed indirectly because of deglacation of North America as the Laurentide retreated some 15.000 years ago: it was created when an ice dam holding back an enormous lake of meltwater collapsed, releasing a huge amount of water downstream toward the Atlantic that carved the river’s path. Past glaciers and glaciations affect civilisation and ecosystem, haunting us (a friendly haunting, I like to think) in ways we’re often not aware of.
An addendum to this section, a related bonus kind of archiving, is the measurement of cosmogenic nuclides. There’s always cosmic rays coming in through our atmosphere, which cause spallation—the fracturing of elemental nuclei, creating, for example, the Beryllium 10 isotope. This is a pretty surficial process, happening within 10s of centimeters of the surface. So when a glacier covers a rock, for example, spallation stops because the cosmic rays don’t make it through the ice. Using cosmogenic nuclides, we can get a sense of how long a rock was buried by ice (or conversely, how recently/how frequently it was exposed to the atmosphere over a certain time period). Yet another record ice leaves us to tell us something about its ebb and flow.
So now you’re familiar with the different ways glaciers are—and create—archives. In a future post, I’ll discuss how artistic and humanities lenses can help us deepen and reframe our understanding of these archives.
Speculative ice core activity
In a recent workshop, I used the archival indicators of isotopes (1), atmosphere (2), and stuff frozen into the ice (3) to set the stage for thinking about the future:
Five hundred years from now, what will scientists will find in ice cores that will form in the next 400 years?
I asked the students to give both a physical indicator (e.g. CO2 concentration , a new kind of genetic signature, or some other chemical lr physical relic) and a corresponding future-historical event, based in part on this infographic from the British Antarctic Survey:
Students described the peak in concentration of PFAS, the signatures of a new kind of chemical called “algorithmic water”, the remains of all the Flix busses, and a single Oreo. It was a fun way to try to give some structure and thought to what the future might hold and what signatures it will leave behind in the (remaining) ice.
Rabbit holes and recommendations
Pay attention to your tactile sympathies— rather than using this for decluttering, use this for loving! touch the things you love! develop emotional relationships with objects! love more waste less!
I heard a story that there are some 800 year old oak trees somewhere in the east of the netherlands, and i wanted to find out if i could visit them. this took me to a list of the oldest trees in europe (at least those that have been measured and added to this website), which didn’t have those trees on it, but nevertheless suggest a different way of choosing how and where to visit
This garden in long island reminded me of this documentary from 1992 about a Spanish artist painting a quince tree, which I started watching because it was at the end of the list of the 250 best films, as ranked by the British Film Institute and is freely available on archive.org
I‘m always delightfully surprised by the newsletter from extrapractice.space, a rotterdam-based collective. The most recent letter was on how people visualize or sense their internal calendars differently. They are always a thoughtful, person-based, and often structurally interesting. this most recent one is not up yet but their archive is worth a meander-through.
If you’re looking for a way to digest the news without instantaneously hitting rock bottom, might I recommend Today in Tabs
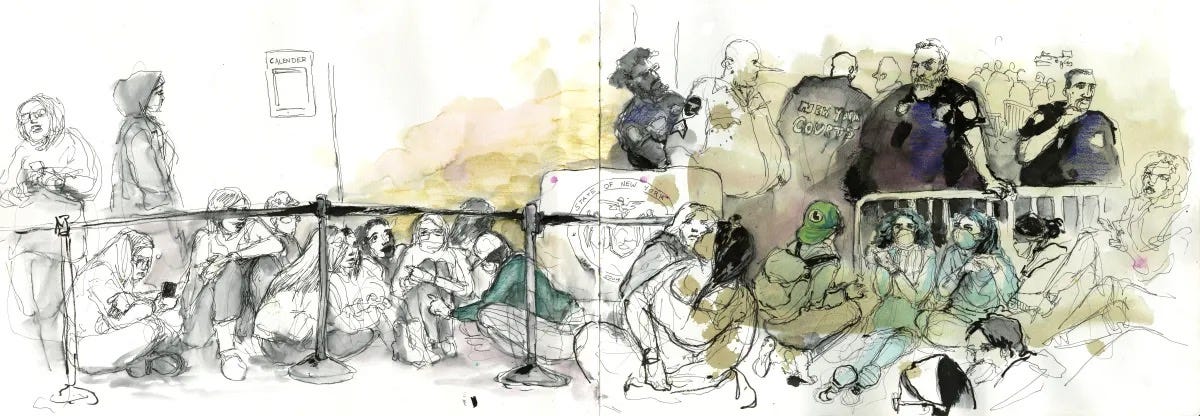
Molly Crabapple on the Jewish Labor Bund and her sketches from Luigi Mangione’s recent hearing in New York
Cheers,
Elizabeth
Thanks for reading! Feel free to subscribe if you aren’t already, or share it with someone who’d love it.




I am excited to follow and subscribe to your Substack. Congratulations on your doctorate.
I loved reading this piece . I also wonder, ( maybe reading too much science fiction), if dormant bacteria long gone could be revived to theoretically harm . If so, couldn’t the opposite be true. Bacteria frozen only to be revived to help cure?